International Market
+300
Products
34
Countries
13
Regulators Approval
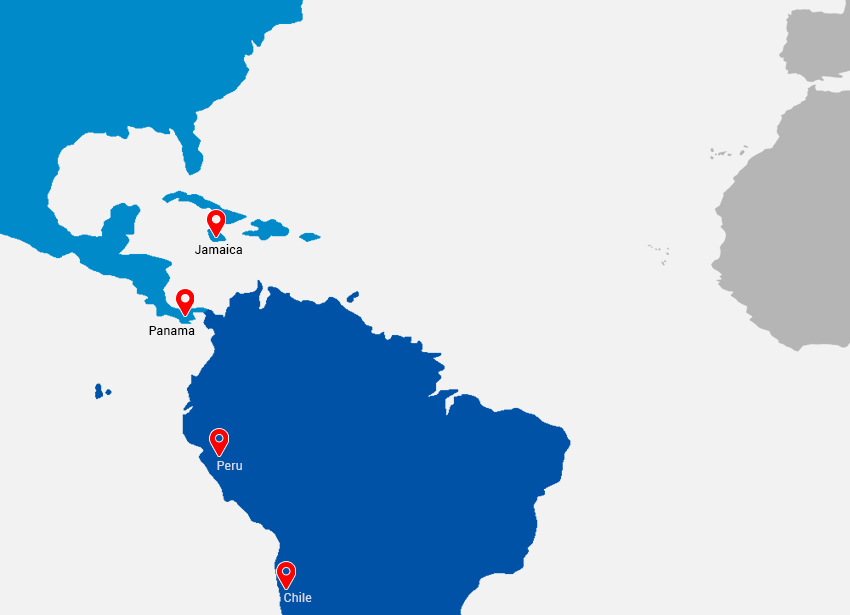
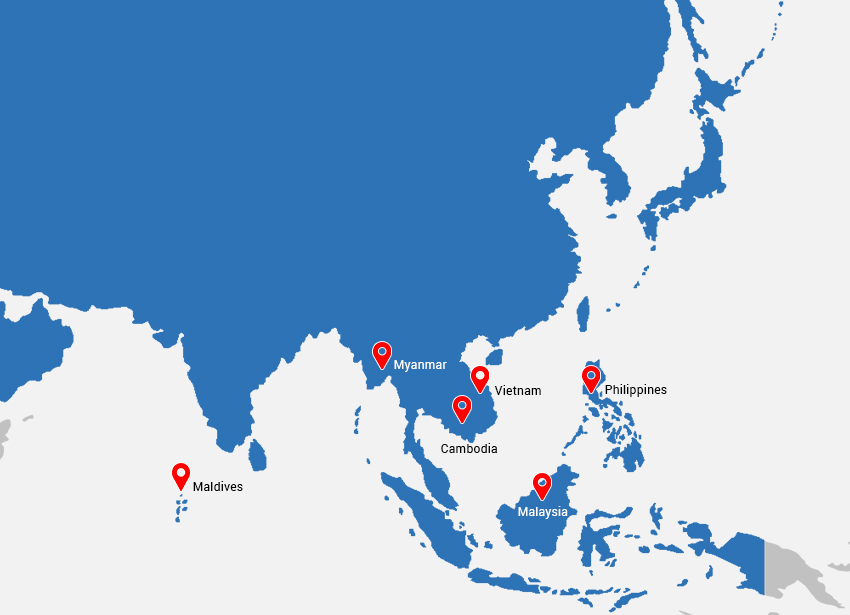
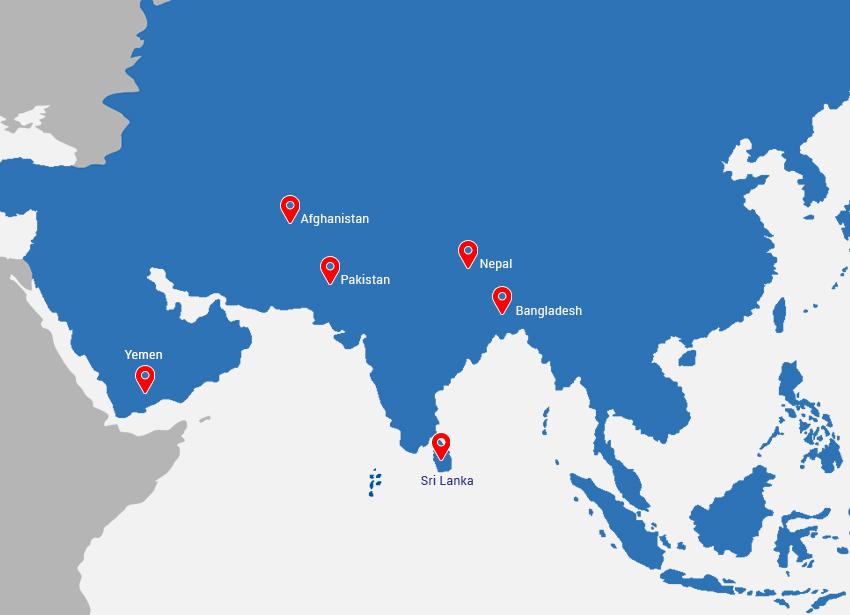
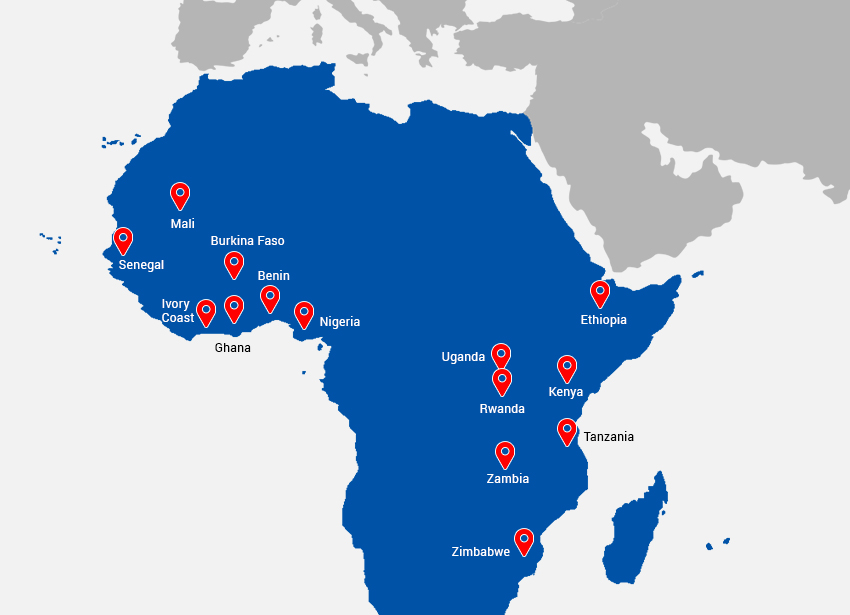
Since its inception, Mankind aspires to be customer-centric and a leader in the Global markets. Committed towards a healthier and happier world, we strive to provide accessible and affordable healthcare to all sections of the society. Today, Mankind operates in 34 countries across the Globe . The company has recently started operations in the markets of CIS , Ukraine , Uzbekistan and Kazakhistan.
Our Presence in Overseas Market
- LATIN: Peru, Chile, Jamaica, Panama
- South East Asia: Philippines, Malaysia, Cambodia &, Myanma
- Asia: Srilanka, Nepal , Maldives , Afghanistan
- Africa: Zimbabwe, Kenya, Tanzania, Zambia, Nigeria, Ghana, Uganda, Rwanda, Ethiopia, Benin, Burkina Faso, Ivory Coast, Mali, Senegal
- CIS: Russian Federation, Ukraine, Moldova ,Kazakhistan & Uzbekistan.
Regulatory Affairs
Our Regulatory Affairs team is highly competent with strategic and tactical experience and works in collaboration with different stakeholders in:
- Designing an appropriate regulatory approach for product development
- Support in generating Right First Time (RFT) documentation for faster approvals & early market launch
- Lifecycle management of registered products & their post-marketing surveillance
- Ensuring regulatory inspections & their maintenance