Recent
Breakthroughs
Breakthroughs
Our clinical and development
pipeline focuses on
pipeline focuses on
New Molecules
Targeting Metabolic Disorders Autoimmune diseases and Oncology with focus on Type 2 diabetes/Obesity/MASH, Allergies and targeted therapy for cancer, respectively.
Research Platforms
Utilizing complex proteins like mAbs, Hormones, peptides, etc., for developing innovative drugs
R&D Pipeline
in Action
in Action
109
New molecules under
development
development
42
New products
launched
launched
Future-focussed
therapeutics
therapeutics
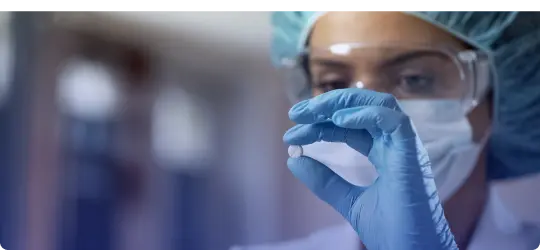
New Drug Discovery Research (NDDR)
Our New Drug Discovery Research (NDDR) division engages in comprehensive drug discovery and development, from conceptualization to IND-enabling preclinical and clinical studies.
Know More 
Biotechnology
We are advancing bio-similar / bio-betters. Our dedicated team of scientists is working on different biosimilar programmes which will soon enter into clinical trials.
Know More 
Research &
Development
Capabilities
Development
Capabilities
Milestones that
make history
make history
Mankind R&D has consistently achieved significant milestones in the pharmaceutical industry.